Inside the Protocol: A Hospital SOP for Dry Chemical Decontamination
In the face of chemical incidents, hospitals are often the first point of contact—before any decontamination protocols have been applied. For regional emergency department staff, this poses immediate risks: volatile chemicals, systemic toxicity, and potential secondary contamination that could impact both patients and staff. A fast, reliable, and adaptable protocol becomes essential.
While wet decontamination has traditionally been used in emergency situations, its limitations in clinical environments are significant. The need for space, low-pressure water mist, and potential contamination of critical care zones makes wet methods increasingly impractical—especially during mass casualty incidents or mass events.
This is where FAST-ACT’s dry decontamination protocol comes in.
The Need for a Dry, Hospital-Based Solution
Hospitals require decontamination procedural approaches that emphasize rapid decontamination, procedural efficiency, and adaptability to tight, high-pressure environments.
The form of decontamination should:
- Enable immediate action
- Minimize risks of exposure to particulate contaminants, nerve agents, and liquid contaminants
- Support continued treatment like life-saving first aid and essential treatment
- Avoid reliance on infrastructure not readily available during a release incident
FAST-ACT meets these criteria with a dry, field-proven approach to emergency decontamination that doesn’t compromise care or safety.
Breaking Down the SOP: How It Works in Real Time
The hospital standard operating procedure (SOP) for FAST-ACT provides a full protocol for hazmat decontamination, including the use of absorbent materials, blotting and rubbing techniques, and tools designed for both patients and medical environments.
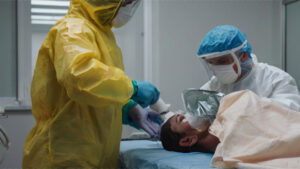
Preparation & PPE:
Before any technical decontamination begins, staff must wear proper protective clothing, and the patient is given a disposable face shield and an N95 or FFP2 mask to reduce contamination from hair and avoid inhaling volatile chemicals. These initial steps are crucial for safe removal and survivor decontamination.
Whole-Body Decontamination:
Using the FAST-ACT 400g Pressurized Cylinder, healthcare providers apply a fine spray across the patient’s full body. This active drying method is dry and immediate, making it ideal in settings without access to water.
The powder contains reactive components that neutralize a wide variety of chemical agents. Importantly, it allows for the removal of contamination without the need for rinsing—perfect for triage or patient transfer situations.
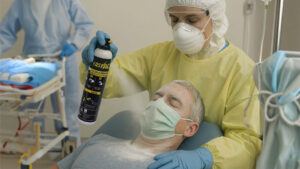
Spot Decontamination:
For exposed skin not covered by clothing, staff may use the FAST-ACT Decontamination Mitts. These mitts utilize adsorbent materials to pat the skin gently, adsorbing and neutralizing any remaining threat. For more sensitive areas, equipment, and skin, the FAST-ACT Decontamination Wipes are used, ensuring compatibility with sterile trauma dressings and sensitive medical instruments.
Environmental Safety and Cleanup:
After the patient is stabilized, the environment may still contain remaining contamination post-exposure. For this reason, the SOP allows for additional cleanup procedures, including HEPA vacuum use and surface wipe-downs. These steps are vital to prevent secondary contamination and support safe use of rooms and corridors post-event.
Trusted by Health Care Providers and Emergency Planners
FAST-ACT’s protocol supports CBRN response plans, meets the needs of responder staffing, and helps coordinate with external agencies such as the poison control center. It provides hospitals with a proven framework to manage technical decontamination during high-stress situations—without halting operations.
FAST-ACT is also built with safety as a priority:
- Non-Toxic Formulation
FAST-ACT’s proprietary powder has been evaluated through a comprehensive toxicity report, confirming it is safe for skin contact and non-toxic via multiple exposure routes. Independent testing shows the product is non-irritating to the eyes, non-sensitizing to the skin, and safe for inhalation within recommended use parameters. This means healthcare providers can perform emergency and technical decontamination with confidence, knowing the process will not add to the patient’s chemical exposure risks.
For access to the FAST-ACT toxicity report, reach out to our team.
- Certified for Skin Use in the EU and Australia
FAST-ACT Decontamination Mitts and Wipes are CE certified in the European Union as a Class I Medical Device for use on skin and carry Therapeutic Goods Administration (TGA) certification in Australia. These certifications confirm compliance with rigorous international safety and performance standards, providing reassurance to hospitals, emergency planners, and health care providers worldwide.
- Proven Compatibility with Wound Care
Recent initial third-party studies have demonstrated that FAST-ACT does not interfere with the natural healing of superficial wounds. This makes it a suitable choice for clinical environments where patients may have injuries in addition to chemical exposure, allowing decontamination to occur alongside ongoing medical care without compromising recovery.
Preparedness and Response: Why It Matters
Preparedness and response are only as good as the tools and protocols available when disaster strikes. Having a dry decontamination plan that integrates seamlessly into hospital intake allows medical teams to act quickly while reducing long-term exposure risks.
FAST-ACT enables:
- Contaminant removal with minimal disruption
- Continuity of care in mass casualty incidents
- Confidence for health care providers managing unknown chemical threats
Every detail in the SOP is crafted to support essential treatment and safe removal in real time.
Final Word
Hospitals must be prepared for the worst while delivering the best. FAST-ACT’s hospital SOP provides a decontamination procedural framework that aligns with today’s chemical threat landscape. Whether you’re facing nerve agents, industrial accidents, or an unknown release incident, the ability to perform dry decontamination safely and efficiently can be the difference between chaos and control.
Download the full SOP below for a detailed guide on implementing FAST-ACT solutions into your mass casualty protocols and hospital emergency response plans or contact our team.
About Timilon Corporation:
Timilon Corporation is the manufacturer of FAST-ACT®, a proprietary formulation of non-toxic high-performance specialty materials effective at neutralizing a wide range of toxic chemicals with the added capability to destroy chemical warfare agents. The FAST-ACT technology is utilized by leading defense agencies, chemical industrial companies, first responders and HAZMAT teams to quickly and safely eliminate chemical hazards. For more information, reach out to Leticia Menzzano, Marketing Manager, lmenzzano@timilon.com.