Hospital Chemical Decontamination Readiness: Why Many Facilities Are Still Unprepared
Hospitals today face increasing risks from hazardous materials, chemical incidents, toxic industrial chemicals (TICs), and chemical warfare agents (CWAs). These events require specialized safety protocols, appropriate Personal Protective Equipment (PPE), and established decontamination protocols to protect both staff and patients. Yet most facilities remain underprepared to manage a chemical release or mass chemical exposure during a mass casualty event.
A recent report revealed that nearly 70% of hospitals are unprepared for chemical and biological emergencies. Additional research supports this trend: many emergency departments lack operational readiness for chemical emergency medical management or hospital patient decontamination.
This blog will cover:
- Why hospital chemical decontamination readiness is difficult to achieve
- What national studies show about preparedness gaps
- How hybrid dry decontamination systems support emergency preparedness plans
- FAST-ACT’s role in chemical decontamination for hospitals
- Actions facilities can take to strengthen emergency preparedness
Understanding the Gap in Hospital Chemical Decontamination Readiness
Multiple studies show hospitals frequently struggle with implementing complete CBRN response plans and meeting NATO standards for chemical incident readiness. For example, fewer than 20% of surveyed facilities had a dedicated plan for hazardous substances or chemical disaster incidents. Only ~45% maintained decontamination units with functioning ventilation or containment for hazardous materials.
Furthermore, only 12% had sufficient respiratory protective equipment for decontamination teams responding to hazmat incidents. A statewide review revealed that 41% of hospitals had no designated decon area setup or decontamination tent. These gaps show that many hospitals still struggle with emergency decontamination operation requirements, including proper training, equipment, and infrastructure.
Why Hospital Chemical Decontamination Readiness Is Hard to Achieve
Water-based decontamination remains a critical element of the patient decontamination process, but it requires substantial infrastructure: heated water storage, water decontamination showers, drainage systems, and dedicated decon corridor spaces. Maintaining this level of capacity year-round is challenging for many facilities, particularly for rural hospitals or facilities without permanent decontamination tents.
Another obstacle is off-gassing, where hazardous substances trapped in clothing or hair continue to release vapors into treatment areas. This creates risks for healthcare workers, particularly when protective equipment cannot fully contain vapor exposure. Immediate mitigation is essential, yet water-based activation often takes time.
Environmental factors further complicate readiness. Cold weather, limited water supply, or power disruptions can delay or stop wet decon operations altogether. Even when infrastructure exists, hospitals may lack regular training exercises to maintain team proficiency. According to CDC research, only 3–10% of hospitals conduct chemical-specific drills. These challenges show why emergency management teams increasingly turn to hybrid decontamination strategies to enhance hospital chemical decontamination readiness.
Hybrid Dry Decontamination: A Modern Best Practice Approach
Dry decontamination systems allow hospitals to initiate safe, immediate chemical removal before water-based systems are active. By neutralizing contamination early, dry decontamination lowers off-gassing, reduces spread into critical areas, and supports first responders and first receivers in maintaining safety protocols under pressure.
Dry decontamination is especially valuable during a chemical release in environments where water-based decontamination cannot be deployed quickly—such as triage zones, ambulance bays, and temporary surge shelters. This method complements traditional water-based decontamination and aligns with CBRN response plans used in modern Disaster Response frameworks. Integrating dry and wet methods provides hospitals with a more resilient system for operational readiness, strengthening their ability to respond during a chemical disaster or large-scale Hazmat Incident.

FAST-ACT: Enabling Effective Hybrid Decontamination in Hospitals
FAST-ACT products support hospital chemical decontamination readiness by providing immediate, validated chemical decontamination solutions. These tools are widely used in emergency management, decontamination tent operations, and across decontamination teams in mass casualty events.
FAST-ACT Pressurized Cylinders
The FAST-ACT Pressurized Cylinder enables rapid powder deployment to neutralize chemicals on clothing, gear, or intact skin. This is particularly important during the first stage of the patient’s decontamination process where hospitals must act before water-based decontamination begins. The cylinder supports hazmat decontamination, reduces vapor hazards, and allows immediate, fast action in decon corridors.
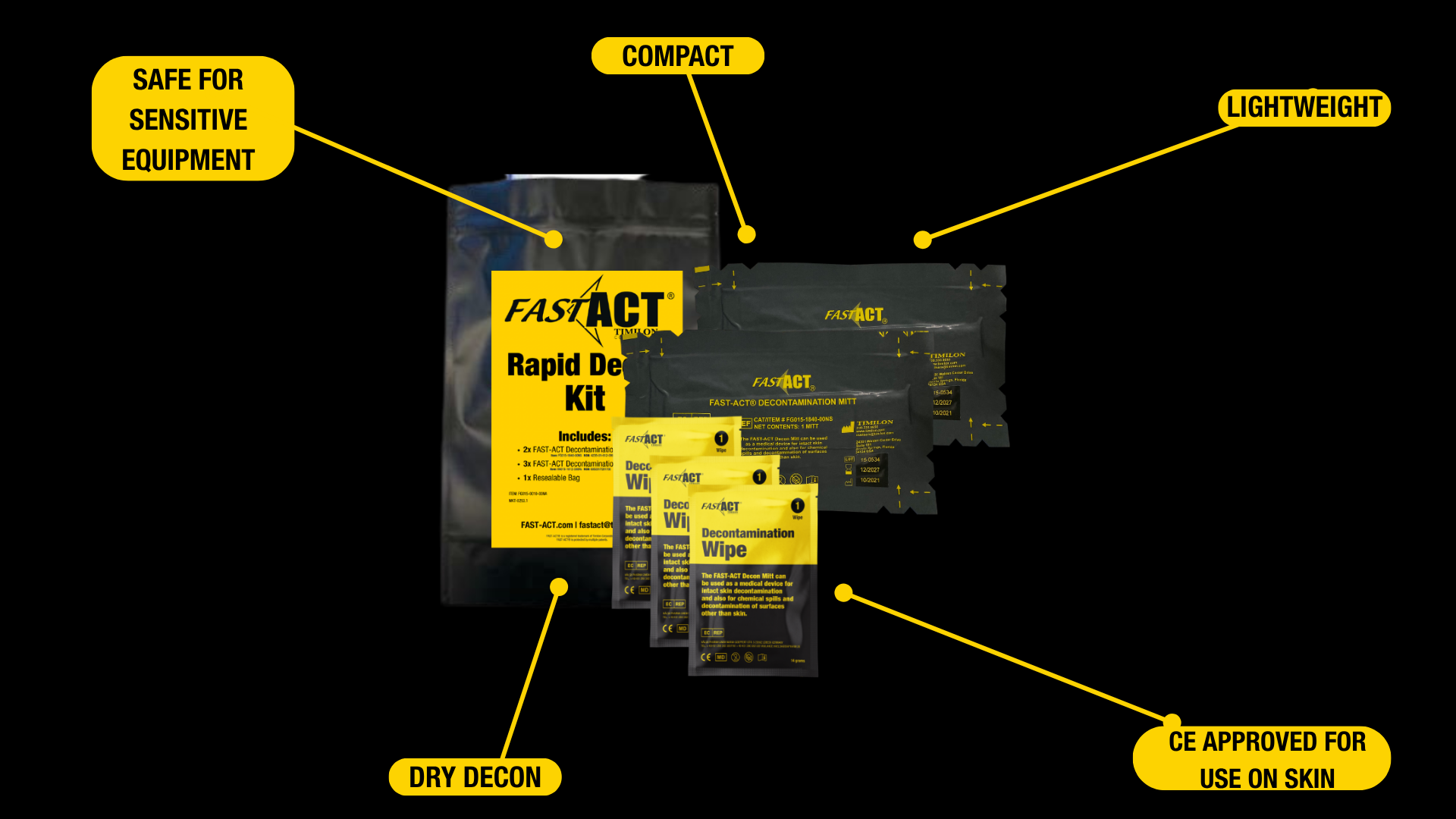
FAST-ACT Decontamination Mitts
FAST-ACT Decontamination Mitts give responders precise manual control for localized dry decontamination. They help remove and neutralize hazardous materials on intact skin and equipment, supporting hospital SOP requirements for chemical emergency medical management and are *CE classified as a class I medical device for use on skin and surfaces in the EU.
FAST-ACT Decontamination Wipes
FAST-ACT Decontamination Wipes gently remove remaining contaminants from sensitive surfaces and intact skin. These wipes support trace removal and operate as part of a complete emergency decontamination operation when used alongside other FAST-ACT tools.
*CE classified as a class I medical device for use on skin and surfaces in the EU.
FAST-ACT Rapid Decon Kit (RDK)
The RDK includes the essential components needed to perform dry decontamination anywhere— particularly useful in mobile decon lines, surge tents, or during large-scale hazardous substances events. The kit includes 2x FAST-ACT Decontamination Mitt, 3x FAST-ACT Decontamination wipes, 1x Seal Bag, allowing emergency management and first receivers to deploy operational tools fast and safely.
Together, these tools support hybrid decontamination workflows, enhance chemical incident safety, and improve hospital chemical decontamination readiness during Chemical Exposure scenarios.
Improving Hospital Chemical Decontamination Readiness: Actionable Steps
Hospitals can strengthen readiness by incorporating chemical training courses that focus on hazmat incidents, reviewing Hospital SOP updates regularly, and ensuring PPE availability for first receiver teams. Conducting training exercises that simulate chemical decontamination or hazardous materials spill specifically prepares staff for real events.
Positioning hybrid dry decontamination tools in triage zones, ambulance bays, and decontamination tents helps teams launch the patient decontamination process faster. Evaluating wastewater management procedures, water-based decontamination capacity, and decon area setup also enhances overall emergency preparedness.
These incremental improvements help hospitals build more robust hospital readiness resources for future events involving hazardous materials or chemical incidents.

Access the FAST-ACT Hospital Blog Series
Conclusion: Strengthening Hospital Chemical Decontamination Readiness
Hospital chemical decontamination readiness remains a challenge across the healthcare sector, with many facilities still lacking essential infrastructure, training, and equipment. Hybrid decontamination systems (dry first, wet second) offer a practical and effective way to enhance disaster response and support safer chemical emergency management.
FAST-ACT’s Pressurized Cylinder and Rapid Decon Kit align with chemical response best practices, helping hospitals improve safety, efficiency, and operational readiness during hazardous materials events. When integrated with water-based decontamination and reinforced through training exercises and safety protocols, hybrid decon forms a stronger foundation for modern hospital preparedness.
For more information on FAST-ACT products or test reports, reach out to our team or visit our training page.
About Timilon Corporation:
Timilon Corporation is the manufacturer of FAST-ACT®, a proprietary formulation of non-toxic high-performance specialty materials effective at neutralizing a wide range of toxic chemicals with the added capability to destroy chemical warfare agents. The FAST-ACT technology is utilized by leading defense agencies, chemical industrial companies, first responders and HAZMAT teams to quickly and safely eliminate chemical hazards. For more information, reach out to Leticia Menzzano, Marketing Manager, lmenzzano@timilon.com.
FAQs
What is the biggest barrier to hospital chemical decontamination readiness?
Limited infrastructure, slow water-based decontamination activation, and limited chemical-specific training exercises.
Why is dry decontamination important for hospitals?
It minimizes chemical spread, reduces off-gassing, and allows immediate action before water decon systems activate.
Is FAST-ACT safe for skin?
FAST-ACT Decontamination Mitts and Decontamination Wipes are CE classified as a class I medical device for use on skin and surfaces in the EU.
Does FAST-ACT replace water-based decon?
No. FAST-ACT supports hybrid systems where dry decontamination precedes water-based decontamination.
Where can FAST-ACT be used inside a hospital?
Common areas include decontamination tents, triage zones, ambulance bays, surge shelters, and decon corridors.